Properties of ionic and covalent compounds lab answer key – Delving into the fascinating realm of chemistry, we embark on a journey to explore the properties of ionic and covalent compounds, unveiling their distinctive characteristics and captivating behaviors. Through this comprehensive guide, we unravel the intricate details of these compounds, their formation, and their unique properties.
Ionic and covalent compounds, the fundamental building blocks of matter, exhibit a remarkable array of properties that shape their physical and chemical behavior. Understanding these properties is essential for comprehending the vast tapestry of chemical reactions and interactions that govern our world.
Properties of Ionic Compounds

Ionic compounds are held together by electrostatic forces between positively charged ions (cations) and negatively charged ions (anions). These forces result from the transfer of electrons from one atom to another, creating oppositely charged ions.
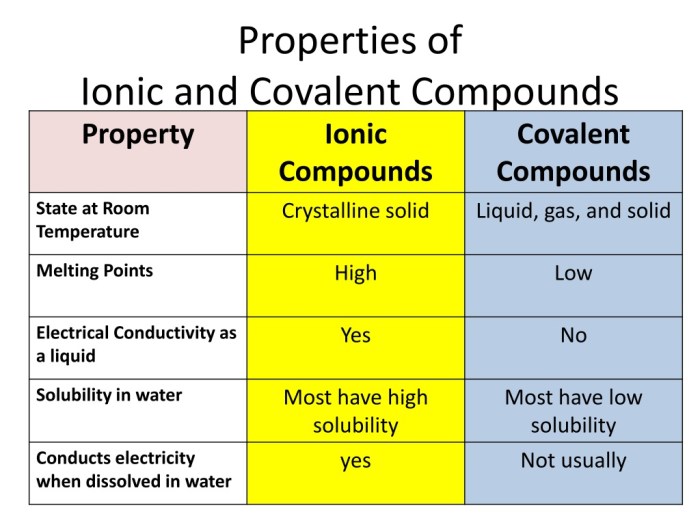
Ionic compounds typically have high melting and boiling points because of the strong electrostatic forces holding them together. They are also soluble in water because the water molecules can surround and separate the ions. Ionic compounds conduct electricity when dissolved in water or melted because the ions can move freely.
Examples of Ionic Compounds and Their Properties
- Sodium chloride (NaCl): high melting point (801°C), high boiling point (1413°C), soluble in water, good electrical conductivity
- Potassium iodide (KI): high melting point (686°C), high boiling point (1330°C), soluble in water, good electrical conductivity
- Calcium oxide (CaO): high melting point (2572°C), high boiling point (2850°C), insoluble in water, poor electrical conductivity
Properties of Covalent Compounds
Covalent compounds are held together by covalent bonds, which are formed when atoms share electrons. The strength of the covalent bond depends on the number of electrons shared.
Covalent compounds typically have low melting and boiling points because the forces holding them together are weaker than the electrostatic forces in ionic compounds. They are also insoluble in water because the water molecules cannot separate the nonpolar covalent bonds.
Covalent compounds do not conduct electricity because the electrons are not free to move.
Examples of Covalent Compounds and Their Properties, Properties of ionic and covalent compounds lab answer key
- Methane (CH 4): low melting point (-182.5°C), low boiling point (-161.6°C), insoluble in water, poor electrical conductivity
- Water (H 2O): low melting point (0°C), low boiling point (100°C), soluble in water, poor electrical conductivity
- Carbon dioxide (CO 2): low melting point (-78.5°C), low boiling point (-56.6°C), insoluble in water, poor electrical conductivity
Comparison of Ionic and Covalent Compounds

| Property | Ionic Compounds | Covalent Compounds |
|---|---|---|
| Bonding | Electrostatic forces between ions | Covalent bonds formed by sharing electrons |
| Melting and boiling points | High | Low |
| Solubility in water | Soluble | Insoluble |
| Electrical conductivity | Good (when dissolved in water or melted) | Poor |
Similarities and Differences
Ionic and covalent compounds both form solids, but ionic compounds are typically harder and more brittle than covalent compounds. Ionic compounds dissolve in water, while covalent compounds do not. Ionic compounds conduct electricity when dissolved in water or melted, while covalent compounds do not conduct electricity.
Examples to Illustrate the Differences
Sodium chloride (NaCl) is an ionic compound that is hard, brittle, and soluble in water. It conducts electricity when dissolved in water or melted. Methane (CH 4) is a covalent compound that is soft, flexible, and insoluble in water. It does not conduct electricity.
Lab Activity: Identifying Ionic and Covalent Compounds
Procedure
- Dissolve a small amount of each compound in water.
- Test the electrical conductivity of each solution using a conductivity meter.
- Record your results.
Materials
- Ionic compounds: sodium chloride (NaCl), potassium iodide (KI), calcium oxide (CaO)
- Covalent compounds: methane (CH 4), water (H 2O), carbon dioxide (CO 2)
- Water
- Conductivity meter
Popular Questions: Properties Of Ionic And Covalent Compounds Lab Answer Key
What is the primary difference between ionic and covalent compounds?
Ionic compounds are formed through the electrostatic attraction between positively and negatively charged ions, while covalent compounds are formed through the sharing of electrons between atoms.
What are some examples of ionic compounds?
Sodium chloride (NaCl), potassium iodide (KI), and calcium oxide (CaO) are common examples of ionic compounds.
What are some examples of covalent compounds?
Methane (CH4), water (H2O), and carbon dioxide (CO2) are examples of covalent compounds.
What are the applications of ionic and covalent compounds?
Ionic compounds are used in various applications, including electrolytes in batteries, fertilizers in agriculture, and pigments in paints. Covalent compounds are found in fuels, plastics, and pharmaceuticals.
